
Ready to Serve!

Ready to Serve!
Electrochemical Cell Chemistry Investigatory Project PDF

Electro Chemical Cell
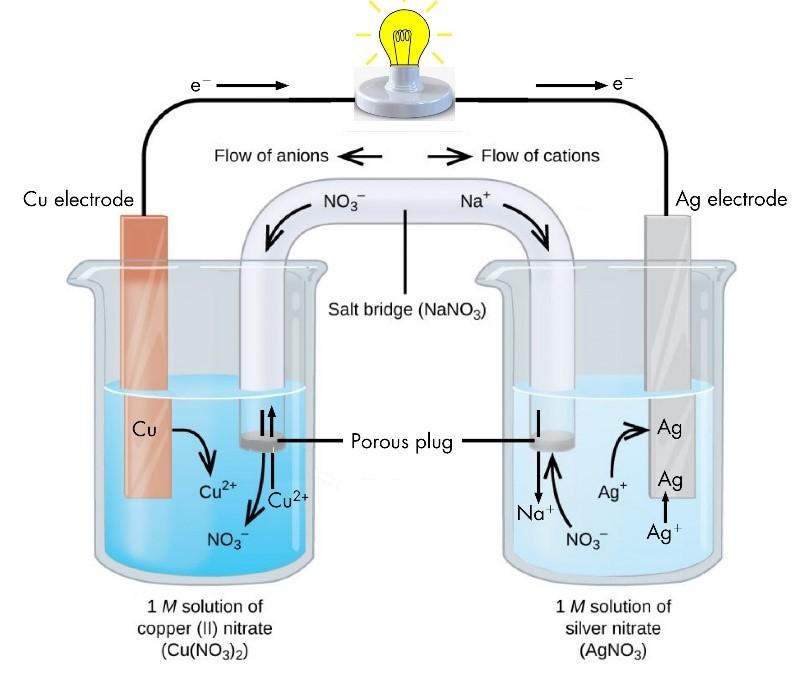
Whenever a redox reaction is allowed to take place directly in a single beaker, it is found that the solution becomes hot. For example, when zinc is placed in a copper solution, the solution is found to be warmer as the reaction proceeds according to the equation.
Zn(s) + CuSO4(aq) —- ZnSO4(aq) + Cu(s)
Similar results are observed when a rod of copper is placed in silver solution. The reaction taking place as follows:
Cu(s) + 2AgNO3(aq) —– Cu(NO3)2(aq) + 2Ag(s)
Thus, we conclude that whenever a redox takes place directly in a single in a single beaker, chemical energy in the form of heat is produced. By suitable means it is possible to bring out the redox reaction indirectly so as to convert the chemical energy into the electrical energy.
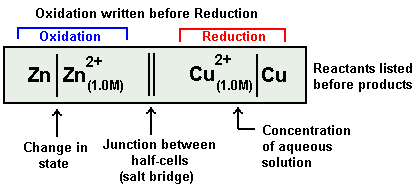
An electrochemical cell is represented in a manner an illustrated below:
Zn | Zn2+ || Cu2+ | Cu
I.e. by convention, the electrode on which oxidation takes place is written on the left-hand side and the other electrode on which reduction takes place is written on the right-hand side. The electrode of the left-hand side is written by writing the symbol of the metal first followed by the symbol of the ion with its concentration in brackets. The electrode on the right-hand side is written by first writing the ion along with its concentration in brackets followed by the symbol of the metal.
A salt-bridge is a U-shaped tube containing concentrated solution of an inert electrolyte like KCl, KNO3, K2SO4 etc.
An inert electrolyte is one whose ions do not take part in the redox reaction and also do not react with electrolyte used. The function of the salt bridge is to allow the movement of the ions from one solution to the other without mixing of the two solutions. Thus, whereas the electrons flow in the outer circuit in the wire, the inner circuit is completed by the flow of ions from one solution to the other through the salt bridge moreover, it helps to maintain the electrical neutrality of the solution of the two half cells.
Thus, the main functions of the salt bridge are:
Let us see what would happen if the salt bridge were not used in the cells show in the following diagram.
Electrons are given out by the zinc electrode where they will neutralize some of the Cu2+ ions of the solution. Thus SO4 2- ions will not leave and the solution will acquire a negative charge. At the same time Zn 2+ ions produced from zinc plate will enter ZnSO4 solution. After some time, the flow of electrons will stop and hence the current stops flowing.
An electrochemical cell is based on reaction which can be split into the two half reactions:
Standard EMF of the cell: Where,
Ecell = Electrode Potential of the cell
Ecathode = Electrode Potential of the oxidation half reaction
Eanode= Electrode Potential of the oxidation half reaction
According to Nernst Equation, the relation between concentration of electrode and the standard electrode potential can be given as:
Ecel = Ecathode – Eanode
E = EO – 0.059/n Log [M]/[Mn+]
Where,
E = Electrode Potential at non-standard conditions
EO = Electrode potential at standard conditions
n = Number of electrons transferred in the equation
[M] = concentration of the metal
[Mn+] = concentration of metal ion
A zinc rod is placed in the zinc sulphate solution taken in a beaker. A copper rod is placed in the copper sulphate solution taken in another beaker. The two rods are connected by a wire and two solutions are connected by a salt bridge.
As shown in below figure
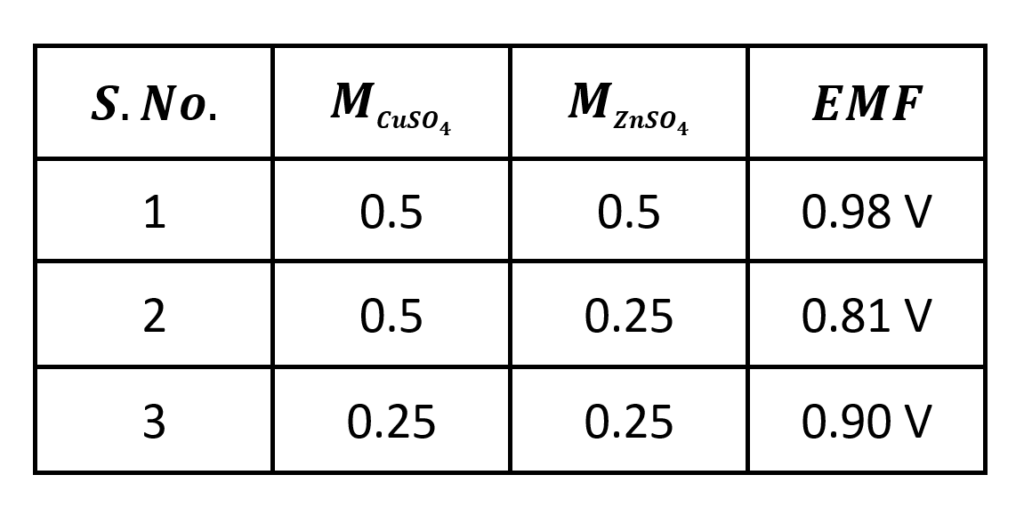
With these observations, we conclude that EMF of the cell increases with decreases in the concentration of the electrolyte around the anode and the increase in the concentration of the electrolyte around the cathode.