To Study the effect of potassium bisulphite as food preservative Project PDF Class 12

Introduction
What are Preservatives?
Growth of micro-organisms in a food material can be inhibited by adding certain chemical substance. However, the chemical substances should not be harmful to the human beings. Such chemical substances which are added to food materials to prevent their spoilage are known as chemical preservatives. In our country, two chemical preservatives which are permitted for use are:
- Benzoic acid
- Potassium hydrogen sulphite
Benzoic Acid or its sodium salt, sodium benzoate is commonly used for the preservation of food materials. For the preservation of fruits, fruit juices, squashes and jams it is used as preservative because it is soluble in water and hence easily mixes with the food product. The efficacy of benzoic acid and benzoate is thus dependent on the PH of the food.
Potassium Bisulphite is used for the preservation of colourless food materials such as fruit juices, squashes, apples and raw mango chutney. This is not used for preserving coloured food materials because sulphur dioxide produced from this chemical is a bleaching agent. Potassium bisulphite on reaction with acid of the juice liberates sulphur dioxide which is very effective in killing the harmful micro-organisms present in food and thus prevents it from getting spoiled.
The advantage of this method is that no harmful chemical is left in the food. The aim of this project is to study the effect of potassium bisulphite as food preservative.
- At different temperatures.
- At different concentrations and
- For different intervals of time
Material Required
Apparatus
- Beaker
- Conical Flasks (100 ml)
- Glass Bottle
- Glass Rod
- Balance Scale
- Knife
- Pestle and Mortar
- Peeler
Chemical
- Sugar
- Potassium Bisulphite
- Fresh Fruits
THEORY
Food materials undergo natural changes due to temperature, time and enzymatic action and become unfit for consumption. These changes may be checked by adding small amounts of potassium bisulphite. The effectiveness of KHSO3 as preservative depends upon its concentration under different conditions which may be determined experimentally
Some Other Methods of Food Preservation are as follows:
- Refrigeration:
One of the most common methods, refrigeration involves storing food at low temperatures to slow down the growth of spoilage microorganisms. It’s effective for short-term preservation of perishable foods like fruits, vegetables, dairy products, and meats. However, it doesn’t halt the spoilage process completely and is not suitable for long-term storage. - Fermentation: Fermentation involves the growth of beneficial microorganisms like bacteria, yeast, or mold, which convert sugars and starches in food into alcohol, acids, or gases. This process creates an acidic or alcoholic environment that inhibits the growth of harmful bacteria. Fermented foods include yogurt, cheese, sauerkraut, kimchi, pickles, and sourdough bread. Fermented foods have enhanced flavors and extended shelf lives.
- Canning:
Canning involves heating food in jars or cans to kill bacteria and other microorganisms, followed by a vacuum-sealing process to prevent recontamination. This method is commonly used for preserving fruits, vegetables, meats, and soups. High-acid foods (pH below 4.6) can be preserved using a boiling water bath, while low-acid foods require pressure canning. Proper processing and sealing are crucial to prevent spoilage. - Drying or Dehydration: Drying removes moisture from food, inhibiting the growth of bacteria, yeasts, and molds that require moisture to thrive. Foods can be dried naturally in the sun or using specialized equipment like dehydrators. Dried foods include fruits, vegetables, herbs, and meats (jerky). Properly dried foods can be stored for extended periods and are lightweight, making them suitable for hiking and camping.
Benefits of Food Preservation
- Preventing Food Spoilage:
Food preservation methods inhibit the growth of microorganisms such as bacteria, yeasts, and molds, which cause food spoilage. This helps to maintain the quality and safety of food products. - Extending Shelf Life:
By slowing down the natural processes of decay, food preservation methods extend the shelf life of perishable food items. This is crucial for reducing food waste and ensuring that food remains edible for longer periods. - Availability of Seasonal Produce:
Preservation techniques allow for the availability of seasonal fruits and vegetables throughout the year. By preserving surplus produce during peak seasons, consumers can enjoy a variety of foods regardless of the time of year. - Convenience:
Preserved foods offer convenience, as they can be stored for extended periods without the need for constant refrigeration or immediate consumption. This is particularly useful for busy individuals or those living in areas with limited access to fresh produce. - Cost Savings:
Preserving food at home or through commercial methods can result in cost savings by allowing consumers to buy food in bulk or take advantage of discounts for seasonal produce. - Food Security:
Food preservation plays a crucial role in ensuring food security, especially in regions prone to natural disasters, where access to fresh food may be limited for extended periods. - Nutritional Retention:
Proper preservation techniques can help retain the nutritional value of foods, ensuring that essential vitamins and minerals are preserved during storage.
Procedure
- Take fresh fruits, wash them thoroughly with water and peel off their outer cover.
- Grind it to a paste in the mortar with a pestle.
- Mix with sugar and colouring matter.
- The material so obtained is fruit jam. It may be used to study the effect of concentration of sugar and KHSO3 , temperature and time.
(A) Effect of concentration of Sugar:
1. Take three wide mouthed reagent bottles labelled as I, II, III.
2. Put 100 gm of fruit jam in each bottle.
3. Add 5.0 gm, 10.0 gm and 15.0 gm of sugar to bottle No. I, II and III respectively.
4. Add 0.5 gm of KNSO3 to each bottle.
5. Mix contents thoroughly with a stirring rod.
6. Close the bottle and allow them to stand for one week at room temperature.
7. Observe the changes taking place in Jam every day.
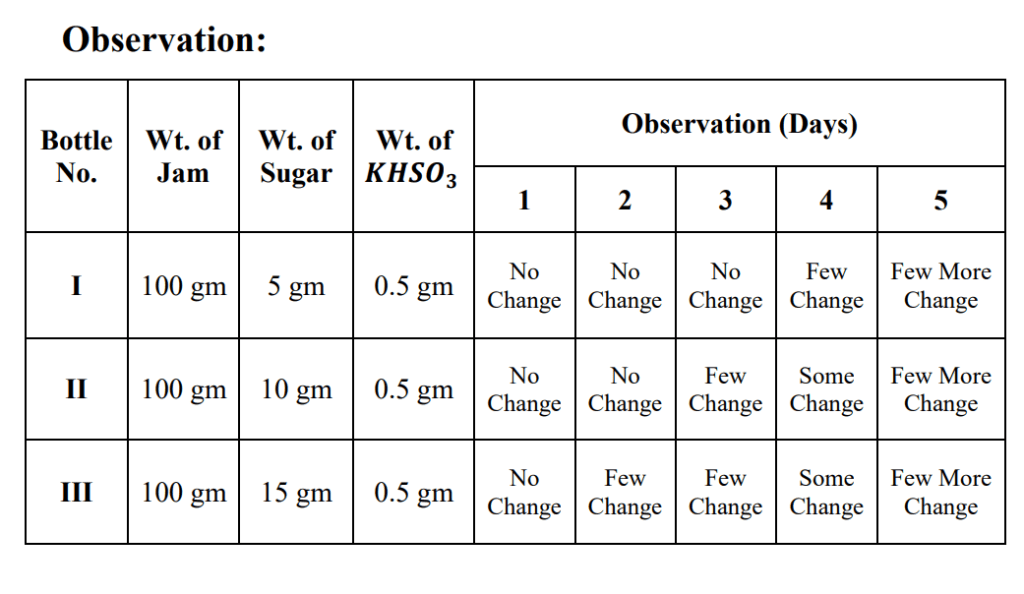
Result:
The increase in concentration of sugar causes fast decaying.
(B) Effect of concentration of KHSO3:
1. Take bottles labelled as I, II, III.
2. Put 100 gm of Jam in each bottle.
3. Add 5.0 gm of sugar to each bottle.
4. Add 1.0 gm, 2.0 gm and 3.0 gm of KHSO3 to bottle No. I, II and III respectively.
5. Mix the contents thoroughly with a glass rod.
6. Keep all the bottles at room temperature for some days and observe the changes every day.
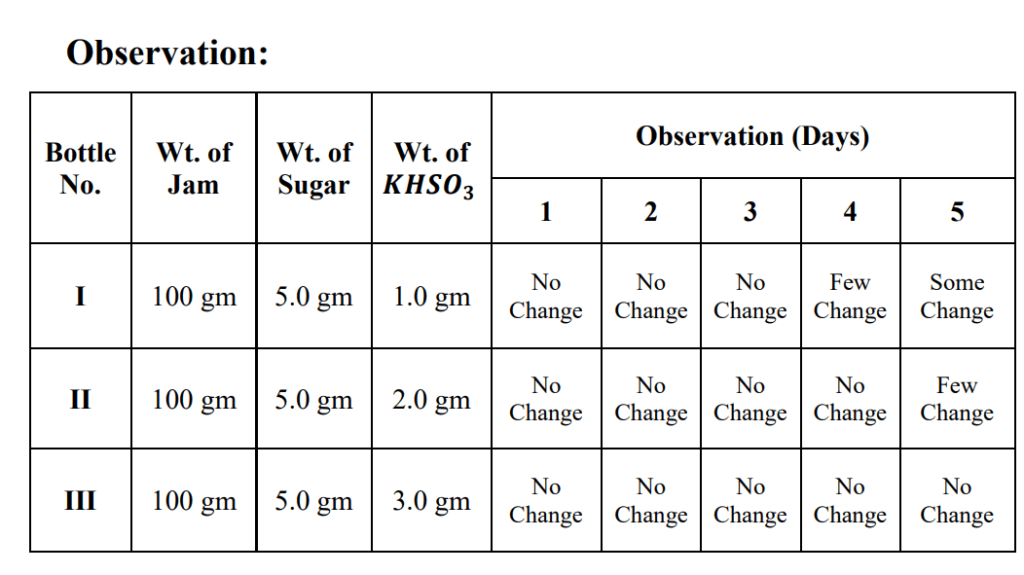
Result:
The increase in concentration of KHSO3 increase the duration of preservation.
(C) Effect of Temperature:
1. Take 100 gm of Jam in three bottles labelled as I, II and III.
2. Add 10.0 gm of sugar and 1.0 gm of KHSO3 to each bottle.
3. Mix the contents thoroughly with a stirring rod.
4. Keep bottle No. I in the refrigerator at 0˚C, bottle No. II at room temperature (25˚C) and bottle No. III in a thermostat at 50˚C. Observe the changes taking place in the jam for some days.
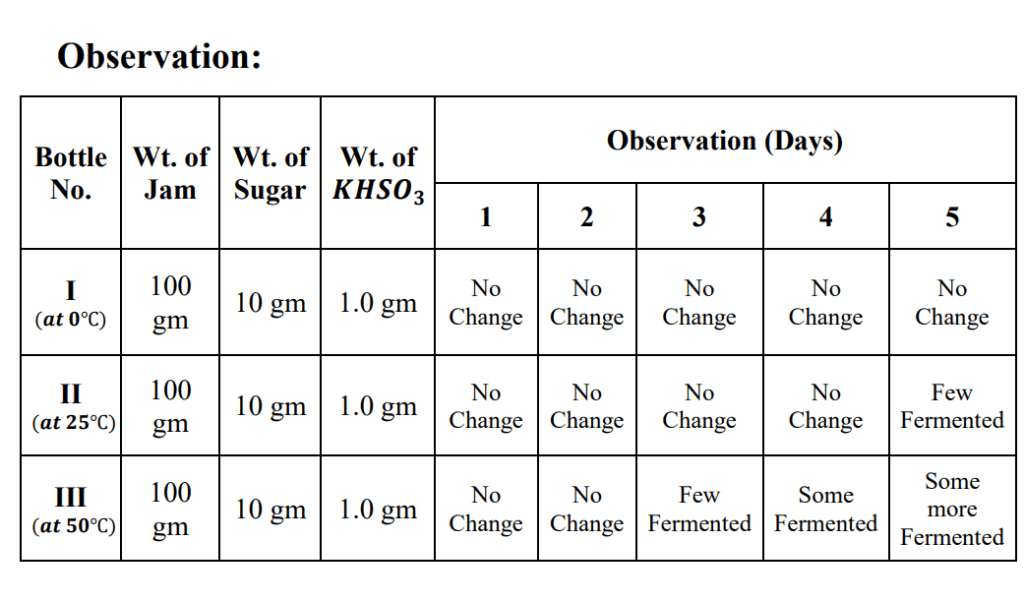
Result:
The increase in Temperature causes fast fermentation of fruit jam
(D) Effect of Time:
1. Take three bottles and label them as I, II and III.
2. To each bottle add 25 g of Jam and 1 g of potassium bisulphite.
3. Keep bottle I for 7 days, bottle II for 14 days and bottle III for 21 days at room temperature.
4. Note the changes taking place in each bottle and record the observations.
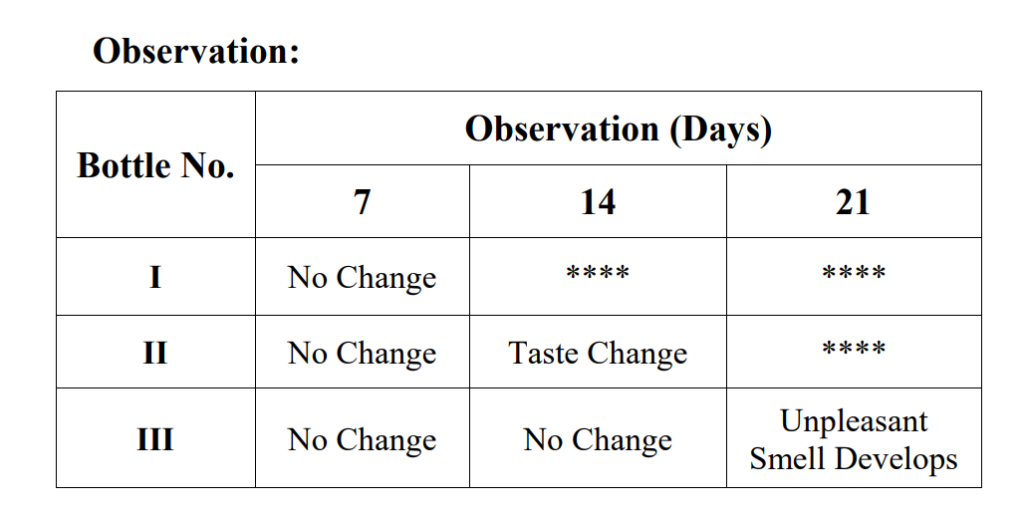
Result:
With increase of days, the quality of the jam deteriorates.
Conclusion
The study aimed to investigate the effectiveness of potassium bisulphite () as a food preservative under various conditions of concentration, time, and temperature. Through a series of experiments, it was observed that the concentration of both sugar and potassium bisulphite significantly influenced the preservation of fruit jam.
Increasing the concentration of sugar in the jam led to faster decay, highlighting the role of sugar concentration in the preservation process. Conversely, higher concentrations of potassium bisulphite resulted in prolonged preservation, indicating its effectiveness in inhibiting microbial growth and preventing spoilage.
Moreover, temperature played a crucial role in the preservation process. Lower temperatures, such as refrigeration, slowed down the fermentation process and extended the shelf life of the jam, while higher temperatures accelerated fermentation, leading to rapid spoilage.
Additionally, the duration of storage also impacted the quality of the preserved jam. Over time, even with the presence of potassium bisulphite, the jam experienced deterioration, indicating the inevitable degradation of food quality over extended periods.
In conclusion, potassium bisulphite showed promise as an effective food preservative, particularly when used in appropriate concentrations and under controlled temperature conditions. However, it’s essential to recognize that preservation methods have limitations, and factors such as time and storage conditions ultimately influence the efficacy of preservation techniques. Further research into optimizing preservation methods and exploring alternative preservatives may contribute to enhancing food preservation practices and reducing food wastage.
Precautions
- Wear appropriate safety gear, including lab coats, gloves, and safety goggles.
- Ensure adequate ventilation in the laboratory.
- Handle chemicals, including potassium bisulphite, with care and follow safety protocols.
- Clearly label all bottles, containers, and apparatuses.
- Use calibrated measuring tools for accurate measurement of chemicals.
- Thoroughly mix ingredients to ensure uniformity.
- Maintain proper temperature control throughout the experiment.
- Regularly observe and record changes in the samples.
- Adhere to strict hygiene practices to prevent contamination.
- Dispose of chemical waste according to safety guidelines and regulations.

