Gene Therapy Biology Investigatory Project PDF Class 12

Abstract
Gene therapy, a transformative branch of medical science, offers unprecedented opportunities for treating and potentially curing a myriad of genetic disorders by manipulating or replacing faulty genes. Grounded in the principles of precision medicine, gene therapy involves the deliberate introduction, removal, or modification of genetic material within a patient’s cells, aiming to address the root causes of diseases at their genetic core. The therapeutic approach encompasses a diverse range of techniques, including the use of viral vectors to deliver therapeutic genes, ex vivo and in vivo strategies, and the revolutionary CRISPR-Cas9 technology for precise gene editing. Recent successes in gene therapy, exemplified by the treatment of conditions such as Severe Combined Immunodeficiency (SCID) and inherited blindness, underscore its potential to revolutionize the treatment landscape. Despite its promise, gene therapy raises ethical concerns, ranging from issues of informed consent and equitable access to the risks associated with germline editing and unintended consequences. Moreover, the high cost of gene therapy introduces challenges related to socioeconomic disparities in access. As the field advances, ongoing research and interdisciplinary collaboration are essential to address these ethical considerations and unlock the full potential of gene therapy, reshaping the future of medicine towards personalized, targeted, and curative approaches.
Genetic Disorder
A genetic disorder is an illness caused by one or more abnormalities in the genome, especially a condition that is present from birth (congenital). They are medical disorders related to gene mutation. Genetic disorders are heritable, and are passed down from the parents’ genes. Other defects may be caused by new mutations or changes to the DNA. In such cases, the defect will only be heritable if it occurs in the germ line. The same disease, such as some forms of cancer, may be caused by an inherited genetic condition in some people, by new mutations in other people, and by non-genetic causes in still other people. These diseases are totally random and difficult to prevent as they are not caused by external agents. Also, as their root cause lies in the genome of the organism their cure was thought to be impossible until the breakthrough research unlocking the secrets of DNA leading to the development of biotechnology and hence gene therapy.
Gene Therapy
We can think of a medical condition or illness as a “broken window.” Many medical conditions result from flaws, or mutations, in one or more of a person’s genes. Mutations cause the protein encoded by that gene to malfunction. When a protein malfunctions, cells that rely on that protein’s function can’t behave normally, causing problems for whole tissues or organs. Medical conditions related to gene mutations are called genetic disorders.
So, if a flawed gene caused our “broken window,” can we “fix” it? What are our options?
- Stay silent: ignore the genetic disorder and nothing gets fixed.
- Try to treat the disorder with drugs or other approaches: depending on the disorder, treatment may or may not be a good long-term solution.
- Put in a normal, functioning copy of the gene: if you can do this, it may solve the problem!
If it is successful, gene therapy provides a way to fix a problem at its source. Adding a corrected copy of the gene may help the affected cells, tissues and organs work properly. Gene therapy differs from traditional drug-based approaches, which may treat the problem, but which do not repair the underlying genetic flaw.

Targets for Gene Therapy
But now a question arises, which disorders or diseases can we target using gene therapy? Many disorders or medical conditions might be treated using gene therapy, but others may not be suitable for this approach. For a disease to be targeted by gene therapy it must satisfy the following conditions:
- The condition must result from mutations in one or more genes
- To treat a genetic flaw, the knowledge of which gene(s) to pursue is absolutely necessary. Also, a DNA copy of that gene available in the laboratory. The best candidates for gene therapy are the so-called “single-gene” disorders – which are caused by mutations in only one gene.
- To design the best possible approach, knowledge about how the gene factors into the disorder is required.
For example:
- Which tissues are affected?
- What role does the protein encoded by the gene play within the cells of that tissue?
- Exactly how do mutations in the gene affect the protein’s function?
Adding a normal copy of the gene should fix the problem in the affected tissue. This may seem like obvious, but it’s not. What if the mutated gene encodes a protein that prevents the normal protein from doing its job? Mutated genes that function this way are called dominant negative and adding back the normal protein won’t fix the problem.
- The gene delivery to cells of the affected tissue must be possible. It depends on:
- How accessible is the tissue? Is it fairly easy (skin, blood or lungs), or more difficult to reach (internal organs)?
- What is the best mode of delivery?
The techniques of biotechnology have made it possible to isolate the required gene in the laboratory and also deliver the gene.
Isolation of Gene of Interest
The first step is to find and isolate the gene that will be inserted into the genetically modified organism. Finding the right gene to insert usually draws on years of scientific research into the identity and function of useful genes. Once that is known the DNA needs to be cut at specific locations to isolate the gene of interest. This can be done by using restriction enzymes also known as molecular scissors which cut DNA at specific sites containing palindromic DNA sequences. But in order to cut the DNA with restriction enzymes, it needs to be in pure form, free from other macro-molecules.
Isolation of DNA
Since the DNA is enclosed within the membranes, we have to break the cell open to release DNA along with other macromolecules such as RNA, proteins, polysaccharides and also lipids. This can be achieved by treating the bacterial cells/plant or animal tissue with enzymes such as lysozyme (bacteria), cellulase (plant cells), chitinase (fungus). Genes are located on long molecules of DNA intertwined with proteins such as histones. The RNA can be removed by treatment with ribonuclease whereas proteins can be removed by treatment with protease. Other molecules can be removed by appropriate treatments and purified DNA ultimately precipitates out after the addition of chilled ethanol. This can be seen as collection of fine threads in the suspension.
Cutting of DNA
Restriction enzyme digestions are performed by incubating purified DNA molecules with the restriction enzyme, at the optimal conditions for that specific enzyme. The cutting of DNA by restriction endonucleases results in the fragments of DNA. These fragments can be separated by a technique known as gel electrophoresis. Since DNA fragments are negatively charged molecules they can be separated by forcing them to move towards the anode under an electric field through a medium/matrix. The separated bands of DNA are analysed for the required gene and then it is cut out from the agarose gel and extracted from the gel piece. This step is known as elution.
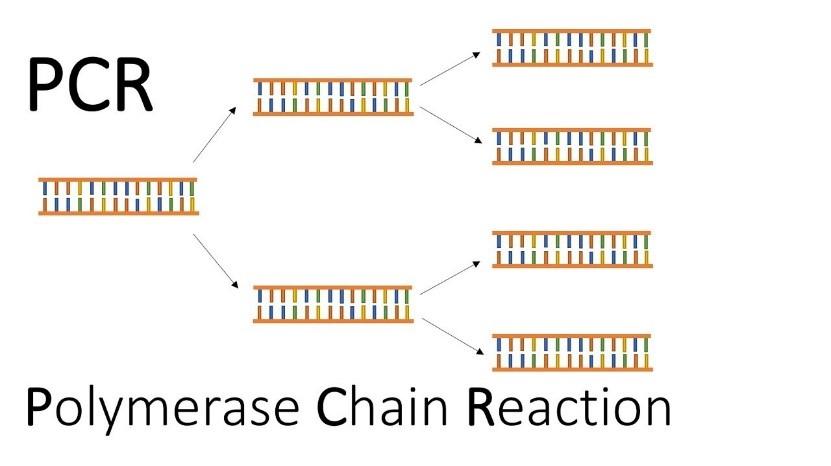
Multiplication of Gene (PCR)
PCR or polymerase chain reaction is then used to create multiple copies of the gene of interest. In this reaction, multiple copies of the gene (or DNA) of interest is synthesised in vitro using two sets of primers (small chemically synthesised oligonucleotides that are complementary to the regions of DNA) and the enzyme DNA polymerase. The enzyme extends the primers using the nucleotides provided in the reaction and the genomic DNA as template. If the process of replication of DNA is repeated many times, the segment of DNA can be amplified to approximately billion times, i.e., 1 billion copies are made.
Gene Targeting
Gene delivery is one of the biggest challenges in the field of gene therapy.
Gene Delivery includes:
- Targeting the right cells.
- Activating the gene. A gene’s journey is not over when it enters the cell. It must go to the cell’s nucleus and be “turned on,” meaning that its transcription and translation are activated to produce the protein product encoded by the gene. For gene delivery to be successful, the protein that is produced must function properly.
- Integrating the gene in the cells. The gene must stay put and continue working in the target cells. If so, it must be ensured that the gene integrates into, or becomes part of the host cell’s genetic material, or that the gene finds another way to survive in the nucleus without being rejected.
- Avoiding harmful side effects. Anytime an unfamiliar biological substance is introduced into the body, there is a risk that it will be toxic or that the body will mount an immune response against it. If the body develops immunity against a specific gene delivery vehicle, future rounds of the therapy will be ineffective.
Choosing The Best Vector
There is no “perfect vector” that can treat every disorder. Like any type of medical treatment, a gene therapy vector must be customized to address the unique features of the disorder. We have learnt the lesson, of transferring genes into plants and animals from bacteria and viruses, which have known this for ages – how to deliver genes to transform eukaryotic cells and force them to do what the bacteria or viruses want.
Part of the challenge in gene therapy is choosing the most suitable vector for treating the disorder. Some vectors commonly used are:
Viruses
Usually when we think of viruses, we think of them causing diseases such as the common cold, the flu, and HIV/AIDS. When faced with the problem of gene delivery, scientists looked to viruses. Why reinvent the wheel if there’s a perfectly good one out there? If we can modify viruses to deliver genes without making people sick, we may have a good set of gene therapy tools.
General advantages of viral vectors:
- They’re very good at targeting and entering cells.
- Some viral vectors might be engineered to target specific types of cells.
- They can be modified so that they can’t replicate and destroy the cell
General drawbacks of viral vectors:
- A virus can’t “expand” to fit a piece of genetic material larger than it is naturally built to carry. Therefore, some genes may be too big to fit into a certain type of virus.
- Viruses can cause immune responses in patients, resulting in two potential outcomes:
- Patients may get sick.
- A patient’s immunity to a virus may prevent him from responding to repeated treatments.
However, modern viral vectors have been engineered without most of the proteins that would cause an immune response.
Non-Viral Vectors
Although viruses can effectively deliver genetic material into a patient’s cells, they do have some limitations. It is sometimes more efficient to deliver a gene using a non-viral vector, which has fewer size constraints and which won’t generate an immune response.
Non-viral vectors are typically circular DNA molecules, also known as plasmids. In nature, bacteria use plasmids to transfer genes from cell to cell.
Scientists use bacteria and plasmids to easily and efficiently store and replicate genes of interest from any organism.
Vectors used at present, are engineered in such a way that they help easy linking of foreign DNA and selection of recombinants from non-recombinants.
These are not the only way to introduce alien DNA into host cells. In a method known as micro-injection, recombinant DNA is directly injected into the nucleus of an animal cell. In another method, suitable for plants, cells are bombarded with high velocity micro-particles of gold or tungsten coated with DNA in a method known as biolistic or gene gun.
Benefits of Gene Therapy
Gene therapy holds the promise of treating and potentially curing a wide range of genetic disorders by introducing, removing, or modifying genetic material within a person’s cells. Some of the key benefits of gene therapy include:
- Treatment of Genetic Disorders:
Gene therapy has the potential to treat and cure genetic disorders by correcting or replacing faulty genes. This includes conditions such as cystic fibrosis, sickle cell anaemia, and muscular dystrophy. - Precision Medicine:
Gene therapy allows for personalized and targeted treatment, as it can be tailored to the specific genetic makeup of an individual. This precision helps minimize side effects and increases the effectiveness of the treatment. - Reduced Need for Ongoing Treatment:
Unlike traditional treatments that may require lifelong medication, gene therapy has the potential to provide a one-time treatment that leads to a lasting or permanent effect. This can significantly improve the quality of life for patients. - Treatment of Incurable Diseases:
Gene therapy offers hope for diseases that currently have no cure. By addressing the root cause at the genetic level, it opens up possibilities for treating conditions that were previously considered untreatable. - Potential for Cancer Treatment:
Gene therapy is being explored as a treatment for certain types of cancer. It may involve modifying a patient’s own immune cells to better target and destroy cancer cells, offering a promising approach to cancer treatment. - Improved Treatment for Neurological Disorders:
Gene therapy is being investigated for treating neurodegenerative disorders such as Parkinson’s and Alzheimer’s disease. It holds the potential to slow or halt disease progression by introducing genes that promote neuronal survival or function. - Treatment of Rare Diseases:
Gene therapy provides a targeted approach for treating rare genetic disorders that affect a small percentage of the population. This can make it economically viable for pharmaceutical companies to develop treatments for these fewer common conditions. - Reduced Side Effects:
Compared to traditional treatments like chemotherapy, gene therapy may have fewer side effects because it aims to specifically target the affected cells without affecting healthy cells. - Advancements in Biotechnology:
The development of gene therapy has driven advancements in biotechnology and genetic engineering. This not only benefits gene therapy itself but also contributes to progress in related fields.
Challenges of Gene Therapy
While gene therapy holds great promise, it also faces several challenges that need to be addressed for its successful and widespread application. Some of the main challenges include:
- Safety Concerns: One of the primary challenges is ensuring the safety of gene therapy. Introducing foreign genetic material into a patient’s cells can lead to unintended consequences, such as triggering an immune response or causing uncontrolled cell division, potentially leading to cancer.
- Efficacy: Achieving consistent and high levels of gene expression in the target cells is essential for the success of gene therapy. Ensuring that the introduced genes are effectively integrated into the patient’s genome and produce the desired therapeutic effect remains a challenge.
- Delivery Methods: Efficient and targeted delivery of therapeutic genes to the specific cells or tissues is a significant hurdle. Developing effective delivery methods that can safely and accurately transport genetic material to the target cells while avoiding off-target effects is a complex task.
- Immune Response: The body’s immune system may recognize the introduced genetic material as foreign and mount an immune response against it. This can reduce the effectiveness of gene therapy and may lead to adverse reactions.
- Cost: Gene therapy treatments can be expensive, limiting accessibility for some patients. The high costs are often associated with the complexity of the technology, research and development expenses, and the relatively low patient numbers for rare diseases.
- Off-Target Effects: Ensuring that gene editing or gene addition occurs only in the intended cells is crucial. Unintended modifications to other parts of the genome, known as off-target effects, can have unpredictable and potentially harmful consequences.
- Long-Term Stability: Sustaining the therapeutic effect over the long term is a challenge. Over time, the introduced genes may lose their effectiveness, or the body’s natural mechanisms may eliminate or silence them.
- Ethical Concerns: Gene therapy raises ethical questions related to the potential for germ-line editing, where genetic modifications can be passed on to future generations. There are ongoing debates about the ethical implications of making heritable changes to the human germline.
- Regulatory Approval: The regulatory pathway for gene therapy is complex, and gaining approval from regulatory agencies involves demonstrating both safety and efficacy. Navigating these regulatory processes can be time-consuming and costly.
- Limited Knowledge of Genetics: Our understanding of the human genome is still incomplete, and many aspects of gene function and regulation are not fully understood. This lack of knowledge can pose challenges in designing precise and effective gene therapies.
Recent Advancements
CRISPR
CRISPR stands for clustered regularly interspaced short palindromic repeats. These RNA sequences serve an immune function in archaea and bacteria, but in the last year or so, scientists have seized upon them to rewrite genes. The RNA sequence serves as a guide to target a DNA sequence in, say, a zygote or a stem cell. The guide sequence leads an enzyme, Cas9, to the DNA of interest. Cas9 can cut the double strand, nick it, or even knock down gene expression. After Cas9 injures the DNA, repair systems fix the sequence – or new sequences can be inserted.
It isn’t the first or only method of gene repair therapy that’s been developed, but the CRISPR technology, says Ramesar, is so special because, unlike previous methods which were more laborious and could only target one kind of cell in the body, it appears to be a “one size fits all delivery”, adaptable for different tissues. The procedure also seems relatively simple to perform.
Ramesar says, from his initial impressions of the literature, that it would seem that localised, accessible abnormal tissue (as in the retina or skin) could be targeted more easily.
Conditions affecting the body more systemically, however, such as certain developmental syndromes, or central nervous system disorders, might be problematic in terms of getting the repair technology into enough of the target cells in that tissue to make an effective difference.
“It may also depend on the stage one attempts to carry out the therapy, in terms of the patient’s age and level of advancement of the disease,” says Ramesar.
Conclusion
Although early clinical failures led many to dismiss gene therapy as over-hyped, clinical successes since 2006 have bolstered new optimism in the promise of gene therapy. These include successful treatment of patients with the retinal disease Leber’s congenital amaurosis, X-linked SCID, ADA-SCID, adrenoleukodystrophy, chronic lymphocytic leukaemia (CLL), acute lymphocytic leukaemia (ALL), multiple myeloma, haemophilia and Parkinson’s disease. These recent clinical successes have led to a renewed interest in gene therapy, with several articles in scientific and popular publications calling for continued investment in the field.

