To Compare the Rate of Fermentation of Wheat Flour, Gram Flour, Rice Flour, Potato Juice, and Carrot Juice Project PDF Class 12

Objective
The purpose of the experiment is to compare the rate of fermentation of the given samples of Wheat Flour, Gram Flour, Rice Flour, Potatoes, Carrot Juice and Orange Juice
I became interested in this idea when I saw some experiments on fermentation and wanted to find out some scientific facts about fermentation. The primary benefit of fermentation is the conversion of sugars and other carbohydrates e.g. converting juice into wine, grains into beer, carbohydrates into carbon dioxide to leaven bread, and sugars in vegetables into preservative organic acids.
Introduction
Fermentation typically is the conversion of carbohydrates to alcohols and carbon dioxide or organic acids using yeasts, bacteria, or a combination thereof, under anaerobic conditions. A more restricted definition of fermentation is the chemical conversion of sugars into ethanol. The science of fermentation is known as zymology. Fermentation usually implies that the action of microorganisms is desirable, and the process is used to produce alcoholic beverages such as wine, beer, and cider. Fermentation is also employed in preservation techniques to create lactic acid in sour foods such as sauerkraut, dry sausages, kimchi and yoghurt, or vinegar for use in pickling foods.
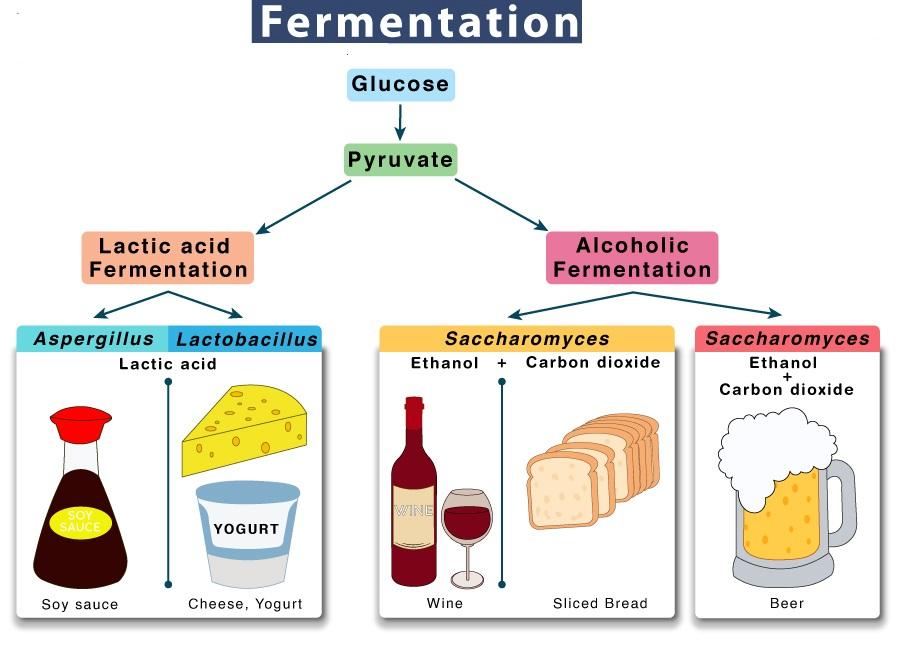
History
Since fruits ferment naturally, fermentation precedes human history. Since ancient times, however, humans have been controlling the fermentation process. The earliest evidence of winemaking dates from eight thousand Years ago in Georgia, in the Caucasus area. Seven thousand years ago jars containing the remains of wine have been excavated in the Zagros Mountains in Iran, which are now on display at the University of Pennsylvania. There is strong evidence that people were fermenting beverages in Babylon circa 5000 BC, ancient Egypt circa 3150 BC, pre-Hispanic Mexico circa 2000 BC and Sudan circa 1500 BC. There is also evidence of leavened bread in ancient Egypt circa1500 BC and of milk fermentation in Babylon circa 3000 BC. French chemist Louis Pasteur was the first known zymologist, when in 1854 he connected yeast to fermentation. Pasteur originally defined fermentation as “respiration without air”.
Contribution to biochemistry
When studying the fermentation of sugar to alcohol by yeast, Louis Pasteur concluded that fermentation was catalysed by a vital force within the yeast cells. He referred to this force as “ferments,” believing they functioned only within living organisms. He stated, “Alcoholic fermentation is an act correlated with the life and organization of the yeast cells, not with the death or putrefaction of the cells.” However, it was later discovered that yeast extracts could ferment sugar even in the absence of living yeast cells.
In 1897, Eduard Buchner, at Humboldt University of Berlin, Germany, demonstrated that sugar fermentation could occur without living yeast cells. He isolated a yeast secretion, which he named zymase, that catalysed the fermentation process. This ground-breaking discovery established the concept of “cell-free fermentation,” for which Buchner was awarded the Nobel Prize in Chemistry in 1907.
Furthermore, studies in 1906 on ethanol fermentation contributed to the early identification of nicotinamide adenine dinucleotide (NAD⁺) as a cofactor involved in cellular metabolism.
Uses
Food fermentation has been said to serve five main purposes:
- Enrichment of the diet through development of a diversity of flavours, aromas, and textures in food substrates.
- Preservation of substantial amounts of food through lactic acid, alcohol, acetic acid and alkaline fermentations
- Biological enrichment of food substrates with protein, essential amino acids, essential fatty acids, and vitamins
- Elimination of anti-nutrients.
- A decrease in cooking times and fuel requirements
Risk of Consuming Fermented Food
Food that is improperly fermented has a notable risk of exposing the eater to botulism. Alaska has witnessed a steady increase of cases of botulism since 1985. Despite its small population, it has more cases of botulism than any other state in the United States of America. This is caused by the traditional Eskimo practice of allowing animal products such as whole fish, fish heads, walrus, sea lion and whale flippers, beaver tails, seal oil, birds, etc., to ferment for an extended period of time before being consumed. The risk is exacerbated when a plastic container is used for this purpose instead of the old-fashioned method, grass-lined hole, as the botulinum bacteria thrive in the anaerobic conditions created by the air-tight enclosure in plastic.
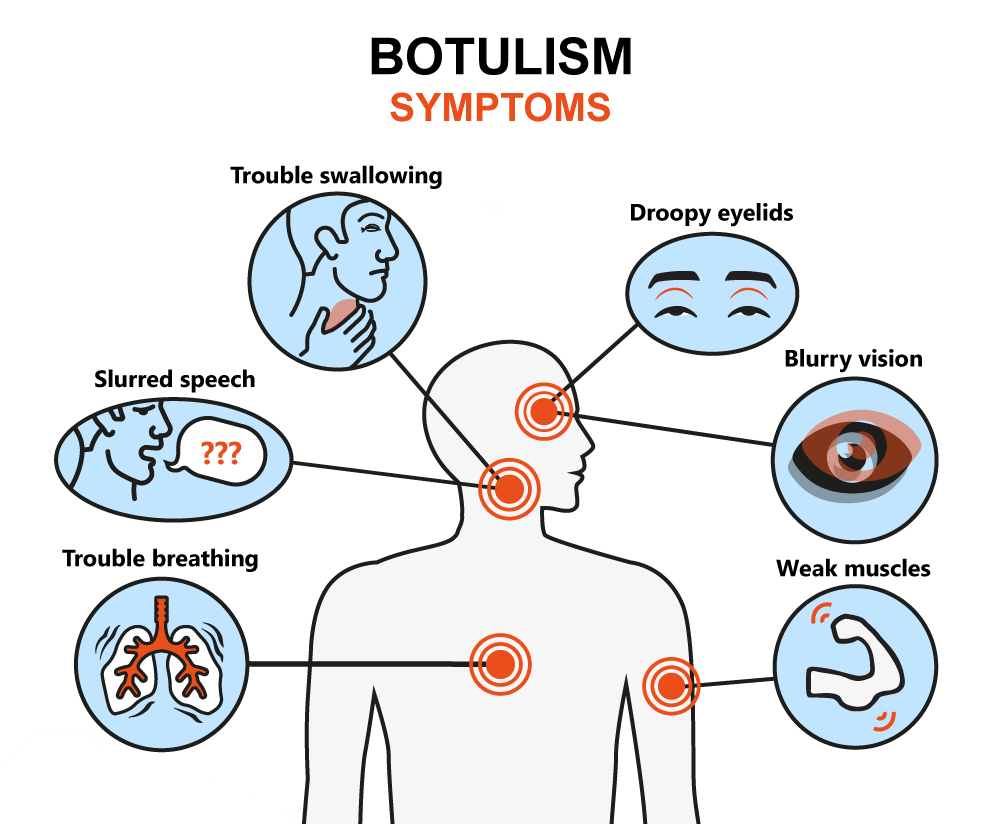
Benefits of Fermented Food Fermented Food
- Enhanced Digestion: Fermented foods are easier to digest because the fermentation process breaks down complex compounds, making it simpler for the body to absorb nutrients.
- Rich in Probiotics: They are a natural source of beneficial bacteria that help maintain a healthy gut microbiome, improving digestive health and reducing gut-related issues.
- Boosts Immunity: Regular consumption of fermented foods can strengthen the immune system by enhancing the gut’s ability to fight off harmful pathogens and inflammation.
- Improved Nutrient Content: Fermentation enhances the availability of essential nutrients, such as B vitamins, vitamin K, and iron, making them more accessible for the body to absorb.
- Better Food Preservation: Fermentation naturally preserves foods, extending their shelf life without the need for artificial preservatives or additives.
- Reduces Inflammation: Fermented foods contain anti-inflammatory properties through probiotics and bioactive compounds, which can help reduce chronic inflammation in the body.
- Aids in Weight Management: By improving gut health and metabolism, fermented foods can regulate appetite, support weight loss efforts, and help maintain a healthy weight.
- Detoxifies the Body: Certain fermented foods can help detoxify harmful compounds in the body, such as phytic acid, and improve the body’s ability to eliminate toxins.
Experiment- 1
AIM-
To compare the rate of fermentation of given sample of wheat flour, gram flour, rice flour and potato using yeast
MATERIAL REQUIRED:
- Conical flask
- Test tube
- Funnel
- Filter paper
- Water bath
- 1 % Iodine solution
- Yeast
- Wheat flour
- Gram flour
- Rice flour
- Potatoes
- Aqueous NaCl solution
Theory
- Take 5 gm of wheat flour in 100 ml conical flask and add 30 ml of distilled water.
- Boil the contents of the flask for about 5 minutes.
- Filter the above contents after cooling, the filtrate obtained is wheat flour extract.
- To the wheat flour extract. taken in a conical flask.
- Add 5 ml of 1% aq. NaCl solution.
- Keep this flask in a water bath maintained at a temperature of
50-60-degree Celsius. Add 2 ml of malt extract. - After 2 minutes take 2 drops of the reaction mixture and add to diluted iodine solution.
- Repeat step 6 after every 2 minutes. When no bluish colour is produced the fermentation is complete.
- Record the total time taken for completion of fermentation.
- Repeat the experiment with gram flour extract, rice flour extract, potato extract and record the observations.
Observation
Sample | Fermentation Time |
Wheat Flour | 10 Hour |
Gram Flour | 12.5 Hour |
Rice Flour | 15 hours |
Potato | 13 hours |
Result
Rice flour takes maximum time for fermentation and wheat flour takes the minimum time for fermentation.
EXPERIMENT-2
AIM-
To compare the rates of fermentation of the following fruit or vegetable juices:
- Orange juice
- Carrot juice
MATERIAL REQUIRED:
- Conical flasks (250ml)
- Test tubes and water bath
- Orange juice
- Carrot juice
- Fehling solution A
- Fehling solution B
- Solution of Pasteur salts and distilled water
Result
- Take 5 ml of orange juice in a clean 250 ml conical flask and dilute it with 50 ml of distilled water.
- Add 2.0 g of Baker’s yeast and 5 ml of Pasteur’s salts to the above conical flask.
- Shake well the contents of the flask and maintain the temperature of the reaction mixture between 35-40°C.
- After 10 minutes take 5 drops of the reaction mixture from the flask and add to a test tube containing 2 ml of Fehling reagent.
- Place the test tube in boiling water bath for about 2min and note the colour of the solution or precipitate.
- Repeat the step 4 after every 10 min. When the reaction mixture stops giving red colour or precipitate with Fehling reagent, the completion of fermentation is indicated.
- Note the time taken for completion of fermentation.
- Repeat the above experiment by taking 5 ml of carrot juice.
Observation
- Volume of fruit juice taken = 5 ml
- Volume of distilled water added = 50 ml
- Weight of Baker’s yeast added = 2g
- Volume of solution of Pasteur’s salts = 5 ml
Result
The rate of fermentation of orange juice is more than the rate of fermentation of carrot juice
Precautions
- Ensure all glassware and apparatus are clean and sterilized to avoid contamination.
- Accurately measure all reagents, yeast, and solutions to maintain consistency.
- Maintain the water bath temperature strictly within the specified range (50-60°C for Experiment 1, 35-40°C for Experiment 2).
- Use freshly prepared fruit and vegetable extracts for reliable results.
- Add yeast and other solutions immediately after preparation to avoid delays in fermentation.
- Perform the iodine and Fehling tests at precise intervals for accurate observations.
- Filter extracts carefully to remove solids and impurities that may interfere with fermentation.
- Cover the flasks properly to prevent external microorganisms from contaminating the samples.
- Conduct the experiment in a well-ventilated area to avoid inhaling any fumes.

